Core Chemistry
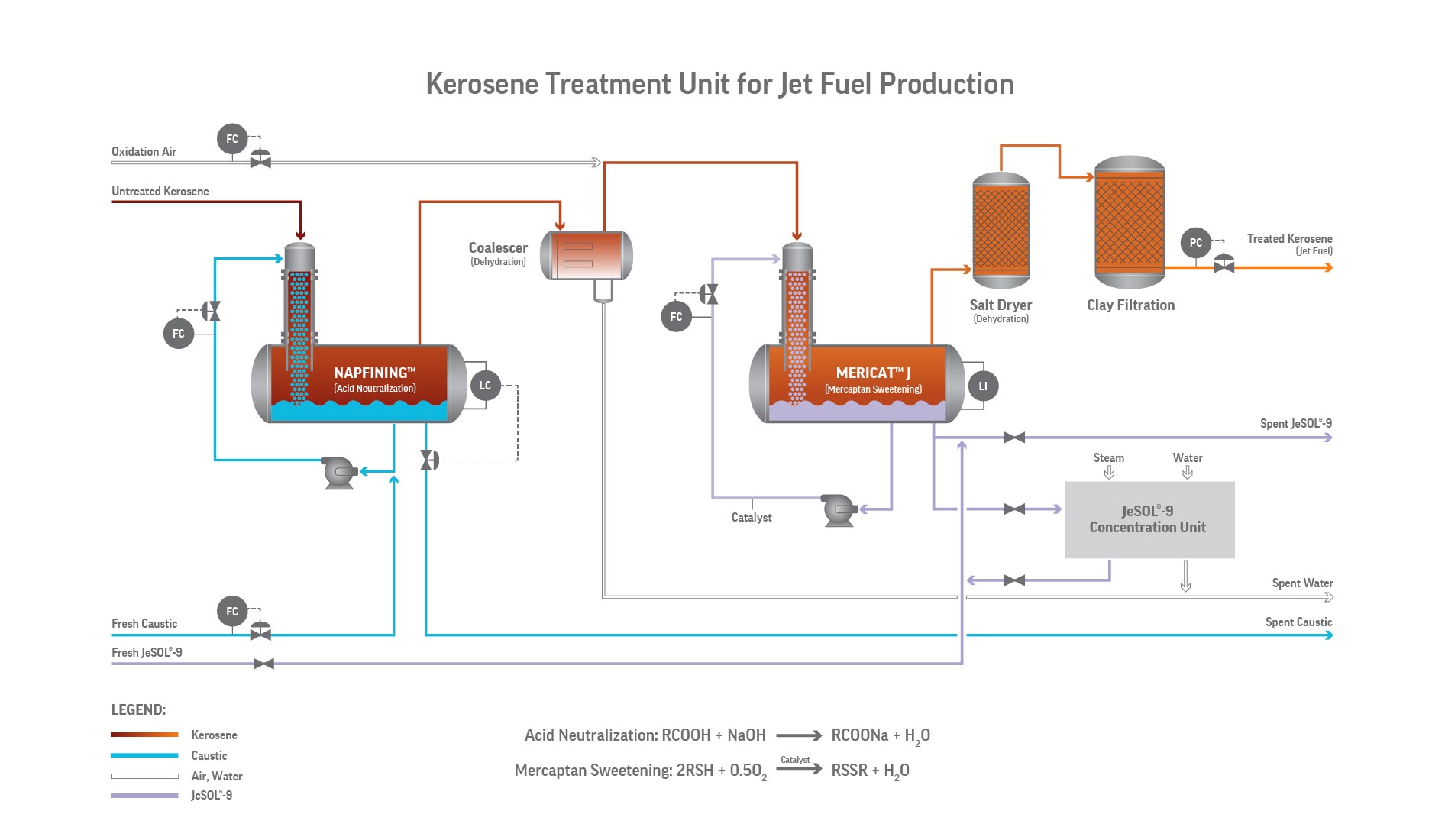
Mericat is an oxidative sweetening process. Mercaptans are first deprotonated in caustic to form mercaptide ions, which are then catalytically oxidized by dissolved oxygen to disulfides:
2 RSH + ½ O₂ → RSSR + H₂O
(overall balanced reaction)
Step-by-step mechanism:
- Extraction / deprotonation: RSH + NaOH → RSNa + H₂O
- Catalytic oxidation: 4 RSNa + O₂ + 2 H₂O → 2 RSSR + 4 NaOH
The catalyst—typically a water-soluble metal phthalocyanine sulfonate (cobalt or vanadium)—cycles between oxidized and reduced states, enabling efficient oxygen utilization and low caustic consumption (typically 0.1–0.5 lb/bbl treated hydrocarbon).
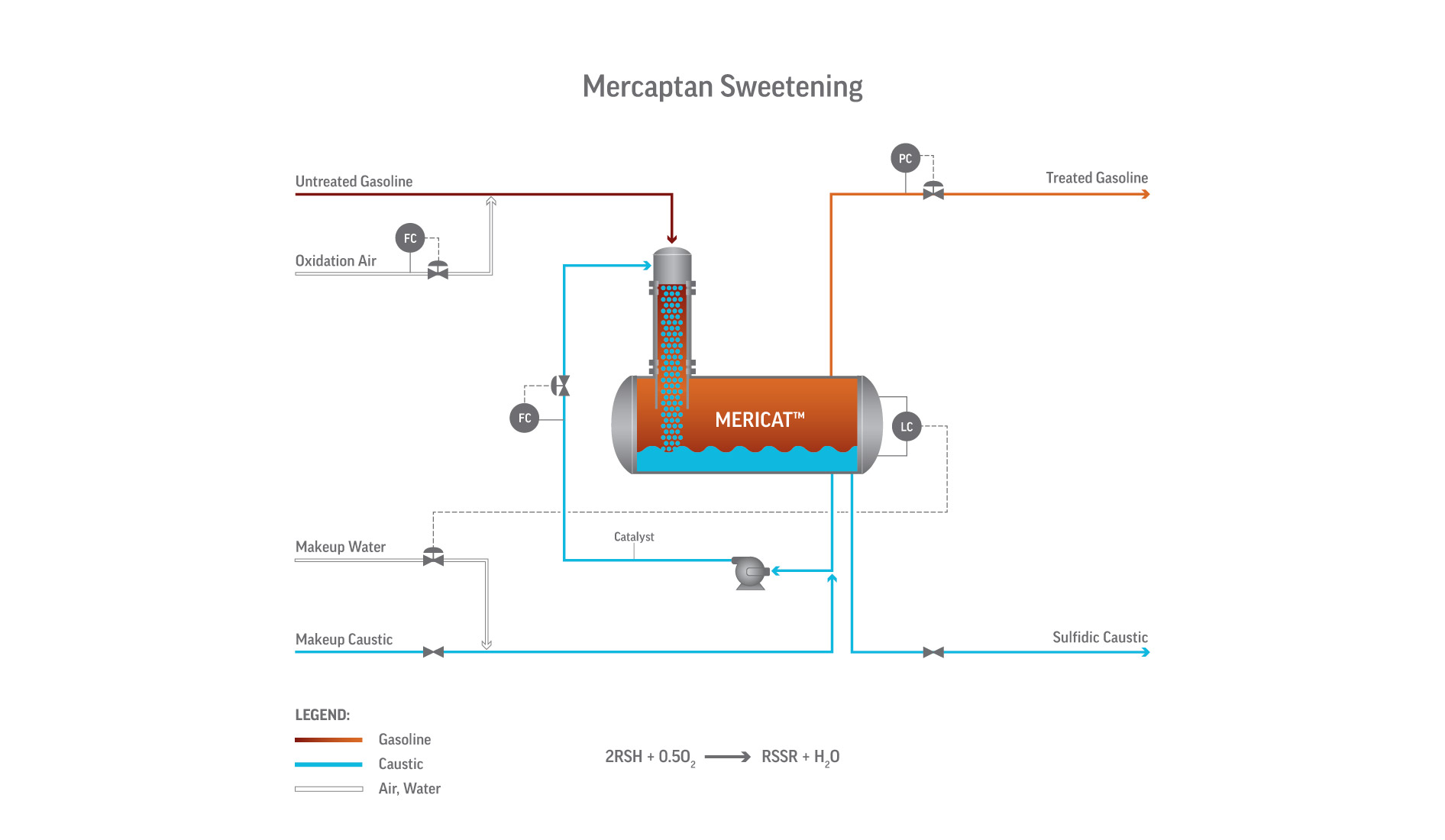
Process Flow and Equipment
The heart of the Mericat unit is the FIBER FILM® Contactor, a bundle of hydrophilic fibers that create an extremely high surface-area film of caustic while the hydrocarbon flows counter-currently. This non-dispersive contact avoids emulsions and allows very efficient mass transfer in a compact vessel.
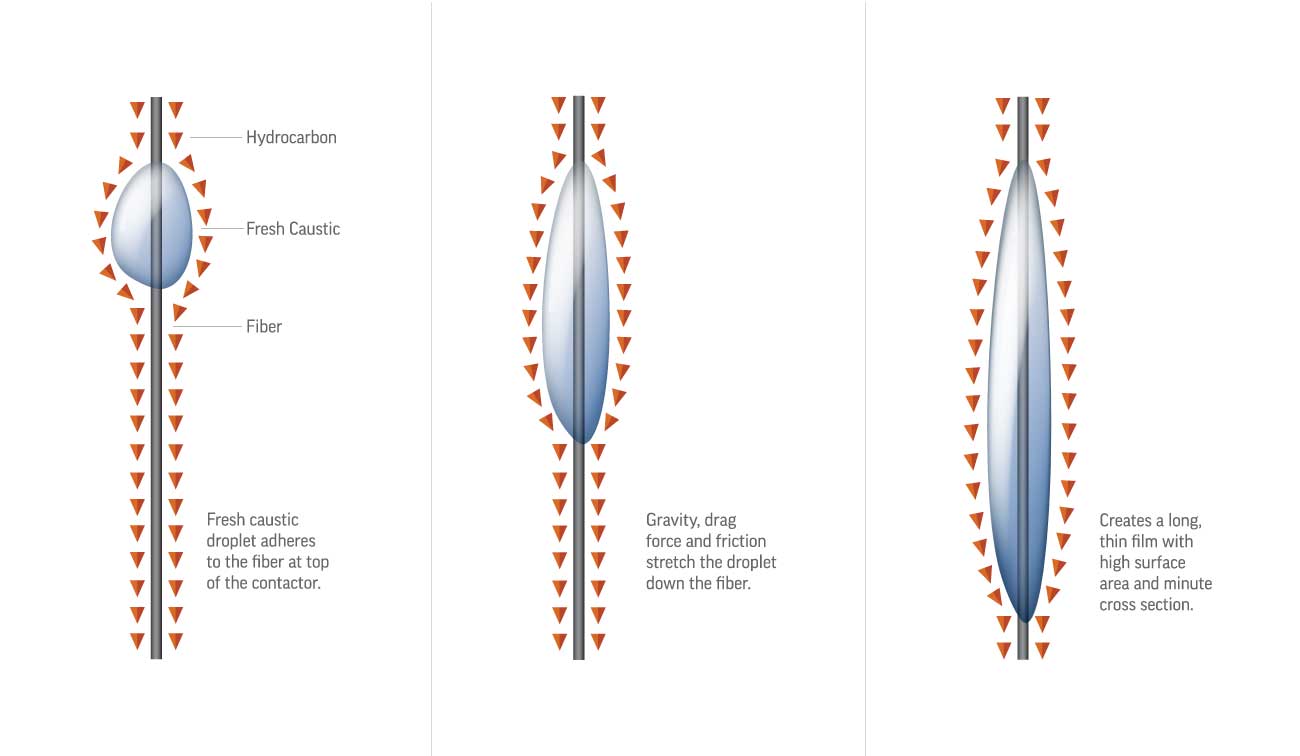
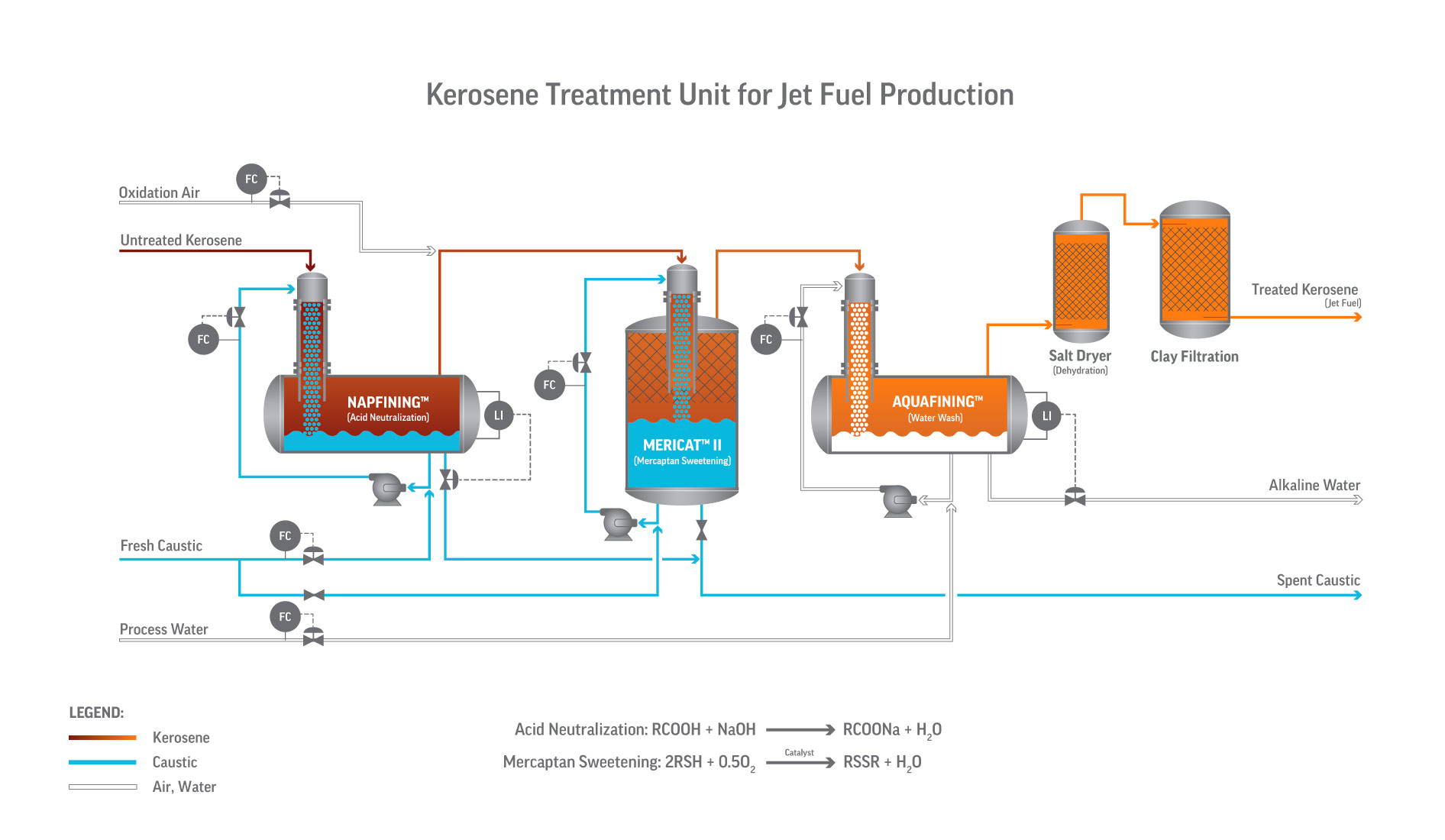
Variants and Application Range
Conclusion
The Mericat process remains a workhorse technology for mercaptan sweetening in liquid hydrocarbons due to its balance of capital efficiency, operating simplicity, and proven performance. For refiners, blenders, and condensate processors in regions like the U.S. Gulf Coast—including facilities near Sugar Land, Texas—Mericat offers a robust, low-maintenance solution to meet odor, corrosion, and specification requirements without the complexity of full hydrodesulfurization.